Our company’s target is to bring to practitioners a range of dental implants with high technology, reliability and safety fundaments in respect of mechanical and biological requirements together with strict cost-control.
Our company’s target is to bring to practitioners a range of dental implants with high technology, reliability and safety fundaments in respect of mechanical and biological requirements together with strict cost-control.
Our products philosophy
DRIVE’s implantary system is developed on following bases :
-
 Cervical bone respect
Cervical bone respect - Soft tissues respect specially in biological distance at the first surgical period, due to the implantary design
- The “dual” aspect means the ability to use the sub-merged and non sub-merged technic (one surgical shot)
- The right placement of prosthetical junctions to the crestal bone
- Cylindro-conical shape implants
- A screw path special design allowing the implant guiding and the primary stabilization even in a poor quality bone (D4 type)
- Rough-porous surfacing due to Corindon surfacing
-
Implantary concept adapted to any surgical procedures :
 Immediate loading
Immediate loading Post-extractional loading
Post-extractional loading Sub-sinusoidal implantology
Sub-sinusoidal implantology Etc...
Etc... - A high performance surgical process due to the “MAGIC” drills and the ream drills
- Simplified prosthetical and surgical kit and packaging
Industrial requirements
 As a manufacturer of dental implants, DRIVE brings a global answer to all practitioners’ clinical cases.
As a manufacturer of dental implants, DRIVE brings a global answer to all practitioners’ clinical cases.
Drive Company is certified ISO 13485 (2003 version) and ISO 9001 as a quality and safety guarantee
 Drive Company manages the whole industrial process from the manufacturing of all products to decontamination, blistering and packaging.
Drive Company manages the whole industrial process from the manufacturing of all products to decontamination, blistering and packaging.
Final Gamma rays sterilization garantees absolute hermeticity and particles-proofing.
 Research and Development
Research and Development
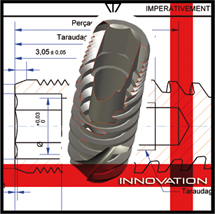
Drive has its own R&D unit which conceives, creates, and ratifies all implantary systems with following targets
- Bio-mechanical validation
- Histological studies on implants interfaces
- Products design validation on following technologies :
Bone Launching Pad™ et Gum Launching Pad™
Patents from December 2006 and March 2008
DRIVE Pilot Centers